Notes On Making Cola
Section 4
Up to: Cube-Cola
| << Section 3 |
4. A Review Of Ingredients
4.1. Alcohol
Alcohol refers to ethanol that has the formula CH3CH2OH, (C2H6O) and has a density of 0.79 g/ml. It is used in some of the recipes to remove the more hydrophobic molecules (terpenes) from the essential oils thus creating a more stable oil/water flavouring emulsion. The alcohol is not later removed from the mixture but once the syrup is completed and subsequently diluted it is present in very small quantities.
The concentration of alcohol in a solution is normally increased by distillation however, it is not possible to get above 95.6% alcohol using this method. For this reason most "pure" alcohol is sold as 95% alcohol. Higher purities of alcohol can be obtained using chemical processes and 100% alcohol is sold as absolute alcohol.
4.2. Benzoic Acid (E210) & Benzoates (E211)
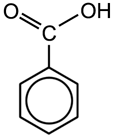
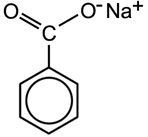
Both of these compounds are added to soft drinks as preservatives. The sodium (Na) salt of benzoic acid is more soluble than the acid (Figure 4.1) itself and is more commonly used. Un-dissociated acid molecules (formed from the salt in solution) are responsible for the anti-microbial action and this is optimum in the pH range 2.5 - 4. These preservatives are effective against yeast but not bacteria and so are usually used in conjunction with sulphur dioxide (see Section 4.14).
 |
 |
|
(a) |
(b)
|
Figure 4.1: Molecules of (a) benzoic acid and (b) sodium benzoate.
4.3. Caffeine
Caffeine is an alkaloid (Figure 4.2) found in cola nuts and numerous other plant matter. It has limited solubility in water and is often added to drinks as the more soluble citrate of caffeine. There is between 70 - 100 mg of caffeine in a cup of coffee and 10g of neat caffeine is enough to kill a person.

Figure 4.2: The caffeine molecule.
Source: Coffee Vol 1: Chemistry, edited R J Clarke & R Macrae (see Bibliography).
4.4. Caramel (E150d)
Caramel colours are amorphous, brown to brownish materials resulting from the carefully controlled heat treatment of food grade carbohydrates in the presence of small amounts of food grade acids, alkalis or salts. They are often liquids with very fine particles suspended in them (colloid suspensions) and have a density of between 1.25 and 1.36 g/ml. Caramel is added primarily for its colouring properties however it will also act as an emulsifier. If caramel is to be the only emulsifier the drops of oil in the emulsion need to be exceptionally small (about 1 micron).
Caramels have a range of pH values and the one that you use should have a low pH (2.5 - 3.5) that is matched to the acidic environment of the cola itself. You should also check that the isoelectric point is lower than pH 1.5 (see Section 1.4.4). Caramels that are appropriate to colas are often called "negative", "acid proof", "class IV" or "ammonium sulphate type" caramels. These caramels are made by heating carbohydrates in the presence of ammonium sulphate and they have negatively charged particles that match the negatively charged droplets in the cola emulsion. Failure to choose an appropriate caramel may cause caramel particles being attracted to the negatively charged oil droplets in the cola emulsion, resulting in a sediment forming at the bottom of the cola. Since the caramels are so acidic care should be taken when handling them.
Source: www.caramelworld.com/solution_center/basics_of_caramel_colors.asp, Beverages by Alan H Varnam and Jane P Sutheralnd (see Bibliography).
4.5. Carbon Dioxide (E290)
Carbon dioxide (CO2) is the only gas suitable for creating fizzy drinks and 2 - 3 volumes are added to the water used to dilute the syrup. Water containing CO2 is acidic and has an odour due to the formation of carbonic acid (H2CO3):
![]()
The carbonic acid is an important acidulant (see Section 3.2.4).
Source: Beverages by Alan H Varnam and Jane P Sutheralnd (see Bibliography).
4.6. Citric Acid (E330)
A carboxylic acid (see Appendix A4) that is abundant in lemons and limes, making up about 8% of their dried weight. It is used extensively in foods and drinks as a preservative and as flavouring. It has a chemical formula of COOHCH2C(COOH)(OH)CH2COOH (Figure 4.3), (C6H8O7) and has a density of 1.665 g/cm3. Normally 1 ½ times the amount of phosphoric acid is added for the same feeling of acidity on the palate. Citric acid is also said to be lighter and fruitier than phosphoric acid.

Figure 4.3: The citric acid molecule.
Source: Beverages by Alan H Varnam and Jane P Sutheralnd (see Bibliography).
4.7. Cola Nut
Also known as guru, goora or bissey nuts. They have a caffeine content of 2.16% which can be extracted by soaking the nuts in alcohol.
Source: "Food Chemistry" by H-D Berlitz & W Grosch (see Bibliography).
4.8 Essential Oils
These oils are a complex mixture of molecules, some of which dissolve more readily in alcohol than others. The exact composition of each oil will vary depending on the source and extraction method. The constituent molecules can be broken down into three categories:
- Hydrocarbons compounds - These are made up from atoms of carbon and hydrogen only. They do not readily dissolve in water but can form an emulsion if an emulsifier is used. Common molecule types include terpenes and monoterpenes (see Appendix A5).
- Oxygenated Compounds - These are organic molecules that contain oxygen. Examples are esters, aldehydes, ketones, alcohols, phenols and oxides. Because they contain polarising oxygen atoms they will be less hydrophobic than the hydrocarbon molecules (see Appendix A4).
- Miscellaneous Compounds - Including acids, lactones, sulphur compounds and nitrogen compounds.
It is the oxygenated compounds that contribute most to the oil's flavour. Different oils will have different proportions of hydrocarbons and oxygenated molecules, therefore different oils will have different solubilities in alcohol. Citrus oils have large amounts of terpenes and are not very soluble in alcohol. However other oils have much larger quantities of alcohols and esters, and are soluble in much smaller volumes of alcohol. Section 4.8.3 summarises the compositions and solubility of the essential oils used in colas.
4.8.1. Flavour Extraction
Extraction processes can be used to remove the hydrocarbon molecules, particularly the terpenes, which have little contribution the flavour and are very hydrophobic. Terpene free essential oils are marketed for use in beverages. As mentioned above (Section 2 and 3.2.1) some cola recipes suggest that the essential oils are mixed in alcohol to remove terpenes, resulting in a mixture that will form a more stable emulsion with water.
Other commercial extraction methods are available including distillation and counter-current extraction. This latter method involves using methanol and hexane; the methanol dissolves the oxygen containing compounds and the hexane dissolves the hydrocarbon molecules, the methanol is easily removed after extraction. Distillation may result in the loss of some of the more volatile flavour components.
Source: Beverages by Alan H Varnam and Jane P Sutheralnd. Common Fragrances and Flavor Materials By Kurt Bauer and Dorothea Garbe (see Bibliography).
4.8.2. Oil Extraction
There are several ways to extract essential oils from plants and the molecular composition of the final oil will depend to some extent on the method used, although many oils can only be extracted by one method:
- Cold Pressing (Expression) - This is probably the most desirable method for flavouring oils but is normally only used for citrus oils. Terpenes are extracted from the plant material by this methods and a subsequent process, such as distillation or counter-current extraction, is needed to make a terpene free oil.
- Liquid CO2 - Liquid CO2 at 10 °C and a very high pressure is used as a solvent to remove the essential oils from plant material. Once at atmospheric pressure the CO2 easily escapes leaving no residue. This process does not remove terpenes and can therefore create a terpene free oil.
- Steam distillation - This is probably the most common extraction method. Steam is used to break down the plant material and the essential oils are released as vapour which is then condensed. This method is not recommended if there is an alternative because insoluble terpenes are extracted and additional terpenes may be created when the plant material is heated. Also some of the more volatile flavour molecules may be lost. Terpenes may be removed from the oil by a subsequent distillation process, however even more of the volatile components may be lost.
- Solvent Extraction - Solvents are used to remove the oils from the plants. This method is not suitable for flavouring oils because solvent residues will taint their flavour.
Source: Common Fragrances and Flavor Materials By Kurt Bauer and Dorothea Garbe, Development In Food Flavours Ed. G G Birch and M G Lindley (see Bibliography).
4.8.3. The Properties Of Essential Oils
Note: Alcohol in the 'solubility' Section refers to ethanol. For more detail about the constituent molecules see Appendix A5. Exact compositions, solubility and densities depend on the country of origin and the extraction method.
Cassia Oil
|
Source:
|
Steam distillation of leaves and young twigs from Cinnamomum cassia (both cinnamon bark and leaf oils are obtained from Cinnamomum zeylanicum). |
|
Composition:
|
Contains up to 90% cinnamaldehyde with lesser amounts of eugenol and other compounds. The composition is similar to that of cinnamon bark oil but cinnamon leaf oil has about 70-90% eugenol and about 7% cinnamaldehyde. |
|
Solubility:
|
1 part in 2, in 80% alcohol. Will form a clear solution in 3 parts 70% alcohol. |
|
Density:
|
1.045-1.065 g/ml @ 25°C |
Coriander Oil
|
Source:
|
Steam distillation of partially dried, fully ripe fruits of Coriandrum stivum. |
|
Composition:
|
Main component is linalool (60-80%). The remainder is mainly terpene hydrocarbons including α-pinene and γ-terpinene (about 5% each). |
|
Solubility:
|
1 part in 8 in 65% alcohol. 2 parts in 1 in absolute alcohol and 1 part in 3 70% alcohol forming a clear solution in the absence of terpenes. |
|
Density:
|
0.863-0.878 g/ml @ 25°C |
Lavender Oil
|
Source:
|
Steam distillation of freshly cut flower tops from Lavandula angustifolia (French lavender oil), Lavandula spica (Spanish spike lavender oil) or a hybrid of the two (lavindin oil). |
|
Composition:
|
Chief components are alcohols and esters. The composition varies considerably depending on the source. French is up to 60% linalyl acetate (ester). Spanish spike has far more linalool. Lavindin is between the other two. |
|
Solubility:
|
Between 1 part in 5, to 1 part in 3 in 70% alcohol, with Spanish spike oil being the most soluble. |
|
Density:
|
0.880-0.895 g/ml @ 25°C |
Lemon Oil
|
Source:
|
Pressed peel of Citrus Limon (lemon petitgrain is from steam distillation of leaves). |
|
Composition:
|
Depends on variety of lemon. Contains mainly terpenes - limonene (about 65%), ß-pinene and γ-terpinene (8-10% each). The lemon flavour is chiefly from citral (aldehydes) (3-10%). |
|
Solubility:
|
Soluble 1 part in 12 absolute alcohol. Probably will not form a clear solution due to wax-like constituents. |
|
Density:
|
0.851-0.855 g/ml @ 25°C |
Lime Oil
|
Source:
|
From whole cold pressed fruit or steam distilled fruit from Citrus aurantifolia. |
|
Composition:
|
Cold pressed: 50-60% limonene and 10% each of ß-pinene and γ-terpinene. The lime flavour come from citral (4.5-9%). The steam distilled oil has 0.5-2.5% citral. |
|
Solubility:
|
Cold pressed: 1 part in 0.5 part 95% alcohol. Steam distilled: 1 part in 5 90% alcohol. |
|
Density:
|
0.910-0.915 g/ml @ 25°C |
Neroli Oil
|
Source:
|
Steam distillation of the flowers of Citrus aurantium (bitter orange tree). |
|
Composition:
|
Depends on the flower source and distillation method but has about 30% linalool. |
|
Solubility:
|
1 part in 2 80% alcohol. |
|
Density:
|
0.868-0.880 g/ml @ 25°C |
Nutmeg Oil
|
Source:
|
Steam distilled from nutmeg, the dried fruit of Myristica fragrans. |
|
Composition:
|
About 90% terpene hydrocarbons. Very similar to mace oil. |
|
Solubility:
|
1 part in 3-4 parts 90% alcohol. |
|
Density:
|
0.862-0.882 (West Indian), 0.883-0.917 (Indonesian) g/ml @ 25°C |
Orange Oil (Sweet)
|
Source:
|
Pressed peels of Citrus sinensis. |
|
Composition:
|
Limonene over 90%. Aldehydes include citral, octanal, decanal. Esters include octyl and neryl acetate. The exact composition depends on the source. |
|
Solubility:
|
Soluble 1 part in 7 parts alcohol. A clear solution will not always form because of the presence of waxy non-volatile substances. |
|
Density:
|
0.842-0.849 g/ml @ 25°C |
Orange Oil (bitter)
|
Source:
|
Pressed peels of Citrus aurantium. |
|
Composition:
|
Limonene over 90%. Less aldehydes and more esters than sweet orange. |
|
Solubility:
|
Soluble 1 part in 7 parts alcohol. A clear solution will not always form because of the presence of waxy non-volatile substances. |
|
Density:
|
0.845-0.851 g/ml @ 25°C |
Source: www.ibiblio.org, Common Fragrance and Flavor Materials by Kurt Bauer and Dorothea Garbe, Analysis Of Essential Oils By Gas Chromatography And Mass Spectrometry By Yoshiro Masada (see Bibliography).
4.9. Glycerine (E442)
Glycerine, also known as glycerin and glycerol, has the chemical formula HOCH2CH(OH)CH2OH and has a density of 1.26 g/ml. It can be obtained from either animal fat or vegetable matter, therefore you should check if you intent to make a vegetarian cola.

Figure 4.4: The glycerine molecule.
Glycerine is added to the cola flavour emulsions as an emulsifier and because it has the combined solvent effects of alcohol and water. It may also act as an anti-oxidant.
Source: Food Flavourings by Joseph Merory (see Bibliography).
Guar Gum (E412)
A polysaccharide gum that can be used to stabilise emulsion.
4.11. Gum Arabic (E414)
Gum arabic is a polysaccharide prepared from the stems and branches of sub-Saharan Acacia senegal and Acacia seyal trees. Unlike other polysaccharide gums molecules of gum Arabic can adsorb to oil droplets in flavour emulsions (see Section 1.5) but has little effect on the viscosity of the water phase in solutions below a 50% concentration. Gum Arabic is soluble in water up to a 50% solution.
In colas it is used as an emulsifier because of its ability to form a protective barrier around oil droplets. Care must be taken to use a gum appropriate for foodstuffs, also there are "emulsion grade" gums that are specially prepared to be used as emulsifiers in beverages. Significant amounts of gum are needed to stabilise cola emulsions; somewhere between 18 and 22% weight/volume. Gum substitutes made from starch are now available.
Source: www.lsbu.ac.uk/water/, www.foodproductdesign.com/ (articles entitled "Pop Art" and "Beverage Stabilizers"), Food Chemistry by H-D Belitz & E Grosch, Polysaccharides In Food by J M U Blanshard & J R Mitchell. (see Bibliography).
4.12. Phosphoric Acid (E388)
A mineral acid with the formula H3PO4 (Figure 4.5). It is used as an alternative to citric acid and is substantially cheaper to produce in bulk. It has been linked with osteoporosis. It is a liquid with a density of 1.834 g/ml (at 18 °C). Normally considered to be flatter and dryer in flavour than citric acid. Phosphoric acid is added at about two thirds of the amount of citric acid to give the same feeling of acidity on the palate.
Phosphoric acid is sold as 50, 75 and 80% strengths.
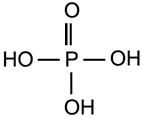
Figure 4.5: The phorsphoric acid molecule.
Source: Beverages by Alan H Varnam and Jane P Sutheralnd (see Bibliography).
4.13. Sugar
Sugars are carbohydrates, therefore they contain carbon, hydrogen and oxygen atoms. Normal granulated sugar is sucrose; a sucrose molecule is made from a glucose molecule and a fructose molecule joined together.
Often some of the sugar is replaced with high-fructose corn syrup (HFCS) and artificial sweeteners. There is a small risk that using HFCS will cause the cola to be contaminated with yeast.
4.14. Sulphur Dioxide (E 220)
Sulphur dioxide (SO2) is added to soft drinks as a preservative and forms sulphurous acid and thus bisulphate and sulphite ions when mixed with water:

Anti-microbial action depends on undisolved sulphurous acid molecules being present, this occurs at low pHs. Sulphur dioxide is effective against all micro-organisms but not some yeasts, it is therefore used in conjunction with Benzoic acid (see Section 4.2).
Source: Beverages by Alan H Varnam and Jane P Sutheralnd (see Bibliography).
4.15. Water
The mineral content of water is important. High levels of minerals such as iron and copper can have an adverse effect on flavour. The stability of emulsions can be reduced if alkaline water (i.e. hard water with a pH greater than 7) is used, therefore calcium and magnesium carbonates should be kept to a minimum. Bottled water is sold with a chemical analysis printed on the label; you should choose the one with the lowest concentrations of Ca+ and Mg2+ ions.
| Parameter | Maximum Permitted Level (mg/l) |
| Total Dissolved Solids | 500 - 850 |
| Alkalinity (as CaCO3) | 50 |
| Chloride | 250 - 300 |
| Sulphate | 250 - 300 |
| Iron | 0 - 0.3 |
| Aluminium | 0 - 0.2 |
Table 4.1: Chemical standards for water for soft drink manufacturers.
In addition to the specifications in Table 4.1 the nitrate level should be kept below 10 mg/l if the cola is to be canned. Also if the water is to be carbonated the level of dissolved oxygen should be below 1 mg/l. Chlorine can be removed from water by passing it through a granulated activated carbon filter or by reverse osmosis.
Source: www.foodproductdesign.com/ (articles entitled "Pop Art" and "Beverage Stabilizers"), Beverages by Alan H Varnam and Jane P Sutheralnd (see Bibliography).
4.16. Xanthan Gum (E415)
Produced by the fermentation of the Xathamonas campestris micro-organism on a glucose medium. Like gum Arabic, xanthan gum is a polysaccharide and it can be used to stabilize emulsions. It acts as a hydrocolloid increasing the viscosity of the water phase and does not act as a surfactant or adsorb to the surface of the oil droplets oil (see Section 1.5.4). About 1% Xanthan gum can produce a surprisingly large increase in viscosity. Xanthan gum is also very stable across a wide range of temperatures and pH.
Source: Polysaccharides In Food edited J M U Blanshard & J R Mitchell (see Bibliography).
| << Section 3 |