Notes On Making Cola
Section 3
Up to: Cube-Cola
| << Section 2 |
3. General Notes On Cola Recipes
3.1. Rumours
Some people who have performed chemical analysis on Coca-Cola claim that it contains the following ingredients: cinnamon, nutmeg, vanilla, glycerine, lavender, fluid extract of guarana, lime juice and other citrus oils. Coca-Cola themselves admit that their drink contains at least 14 ingredients. There are some rumours that coca-cola had to announce to some Muslim countries (including Morocco) that the glycerine they used was produced from vegetable matter and not from pig fat.
3.2. Cola Emulsions And Emulsifiers
Cola is a water-based drink although it is flavoured with oil. This presents a problem since oil and water do not mix and an emulsifier is needed to create a stable beverage. If the cola has not been sufficiently stabilised the oil and water may separate during storage. Originally coca-cola was not intended to be bottled and stability was less of an issue. The original recipe (Section 2.1) does not contain an effective emulsifier, although alcohol and caramel will have some positive effect.
3.2.1. Alcohol Flavour Extraction
The method from the original recipe (Section 2.1) instructs that the oils should be mixed with ethanol (alcohol) before a little water is added. Essential oils are a complex blend of hydrocarbons and oxygenated organic compounds (see Sections 1.1, 1.2 and 4.8). The hydrocarbons have no polarity, will not interact with the alcohol and will separate once the water is added. The compounds that contain oxygen will interact with the alcohol and form a more stable emulsion with the water. Thus this process creates two phases, a flavour extract (clear part) and a washed oil (cloudy part); the flavour extract is used in the cola.
Treating the oils with alcohol will affect the flavour slightly since some of the molecules in the oils are removed. However, the hydrocarbon molecules that are removed (such as limonene and other terpenes, see Appendix A5) do not contribute much to the characteristic flavour of citrus oils. The alcohol extraction has a marked affect on the emulsion stability because the molecules that are removed are the most hydrophobic, and therefore the ones that are most likely cause the emulsion to breakdown. Also the hydrocarbon molecules will not contribute to the surface charge on the oil droplets reducing the effects of electrostatic stabilisation.
The recipe in Section 2.6 specifies that the essential oils are "terpene free". Alcohol extraction will remove these compounds however, there are other extraction methods that do not require alcohol (see Section 4.8.1 and 4.8.2 for details). It seems unlikely that coca-cola sold in Muslim countries would be with prepared using alcohol.
3.2.2. Emulsifiers
Several ingredients can be added to give the cola a longer term stability. Coca-cola seems certain to contain glycerine however, pepsi-cola's website does not list glycerine as an ingredient in any of their beverages. Gum Arabic is commonly used as an emulsifier in colas and is included on Pepsi's ingredient list. New gum Arabic substitutes made from starches are available for use in soft drinks and may be easier to mix.
Caramel, added to cola primarily for its flavour and colour, can also act as an emulsifier. However, it can only stabilise an emulsion of very fine oil droplets.
Weighting agents can also be added to help prevent the oil and water phases separating (see Section 1.5.5). These substances are oils with unusually high densities (greater than the density of water). They are mixed with the flavouring oils so that the overall density of the oil phase is the same as the density of water (1 g/ml). Weighting agents therefore stop the oil droplets, contained within the emulsion, migrating to the surface under the influence of gravity. A common weighting agent is brominated vegetable oil (BVO), however this appears to be banned in Europe and only contained within beverages manufactured in the USA. Even in the USA the amount of BVO that can be added to a beverage is strictly limited.
Glycerol ester of wood rosin (E445, extracted from the stumps of pine trees) is another weighting agent that is sometimes used in citrus flavoured drinks. There are some concerns about the affects that it has on several organs within the human body, it is therefore limited to less than 100 ppm within soft drinks. Other weighting agents suitable for citrus based drinks, such as sucrose acetate isobutyrate, are available from chemical companies.
The densities of various flavouring oils are shown in Section 4.8.3, most are in the range 0.85 g/ml to 0.90 g/ml. An exception is cassia oil, which has a density slightly greater than the density of water; therefore adding more cassia oil will increase the density of the oil phase and increase the stability of the emulsion.
3.2.3. Mixing
Several industrial machines are widely used to mix food emulsion. Normally these use a shearing action to break large droplets up, rather than a simple stirring action (Figure 3.1).
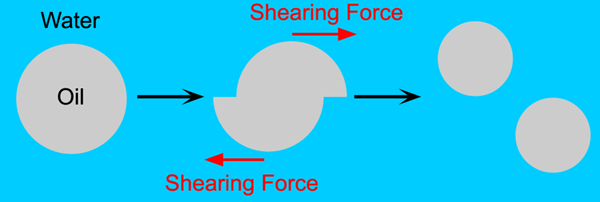
Figure 3.1: The shearing of large oil droplets.
Pressure homogenisers force liquid through a valve at high velocities (50 - 200 ms-1) and pressures up to 10 000 psi (69 MNm-2). The valve gap is about 15 - 300 microns and oil drops in the size 0.1 - 0.2 microns [7] are formed. Two-stage homogenisers force the liquid through a second valve at a lower pressure (400 - 500 psi, 2.8 - 3.4 MNm-2) to break up any droplet flocs that form exiting the first value. These machines tend to be quite large and use several pistons to provide the pressure.
Colloid mills consist of two concentric discs separated by a gap of 50 - 150 microns. One disc remains stationary while the other spins at 3000 - 10 000 rpm. The emulsion is sheared between the discs producing droplets that are 1 - 2 microns in size. Colloid mills tend to be much smaller than pressure homogenisers.
The mixing of the emulsifier with the other ingredients is critical. Often the gum Arabic is first mixed with a small amount of water before it is added to the flavouring oils. Other ingredients, such as caramel and citric acid are usually added as solutions to insure that they mix in easily. For stable emulsions it is usually estimated that the oil droplets should be no bigger that 3 microns, however if a good emulsifier is not used the droplets should not exceed 1 micron.
3.2.4. Acidulants
The sugar/acid balance in colas is very important for "mouth feel" and taste. Phosphoric acid or citric acid are added to create acidity (see Sections 4.6 and 4.12). Citric acid is said to have a light and fruity character, whereas phosphoric acid is said to have a flat, drier flavour. In addition to these acids the carbon dioxide (CO2) in carbonated drinks also acts as an important acidulant; creating carbonic acid in water (see Section 4.5).
3.2.5. Antioxidants
Oil based flavourings are vulnerable to deterioration by oxidation because air becomes trapped in the emulsion during the mixing process. Colas don't seem to have antioxidants routinely added however, other soft drinks contain various chemicals to prevent oxidation. These include compounds that contain tocopherols .
3.2.6. Preservatives
Carbonated soft drinks can support the limited growth of micro-organisms. Acidulants provide some protection since a lot of micro-organisms are not tolerant to acidic conditions, however such substances are considered to be a safe guard and not a primary barrier to microbial growth. Other substances such as sulphur dioxide (SO2) and benzoic acid can be added as preservatives (see Sections 4.2 and 4.14). These two substances are normally added together because they have a synergistic effect; SO2 acts against micro-organisms and benzoic acid guards against yeasts.
3.2.7. Foaming Agents
Foaming agents are added to soft drinks so that a good head is formed on pouring. Saponins from bark of Quillaia or yucca trees are often included to fulfil this purpose.
| << Section 2 |
[7] 1 micron = one 1000th of a millimetre. [Return To Text]