Notes On Making Cola
By
Richard Grove
July 2005
Disclaimer:
This document is copyright free. However you use any information contained within it at your own risk.
Contents
2.2. Another Supposed Original
2.3. The Supposed Pepsi-Cola Recipe
2.5. Stevia Natural Sweetener Sugar Free Cola)
3. General Comments On Cola Recipes
4.6. Glycerine (glycerol, propane-1,2,3-triol, E442)
4.8. Citric Acid (E330)
Roughly speaking molecules can be split into two basic types:
Non-polar
Polar
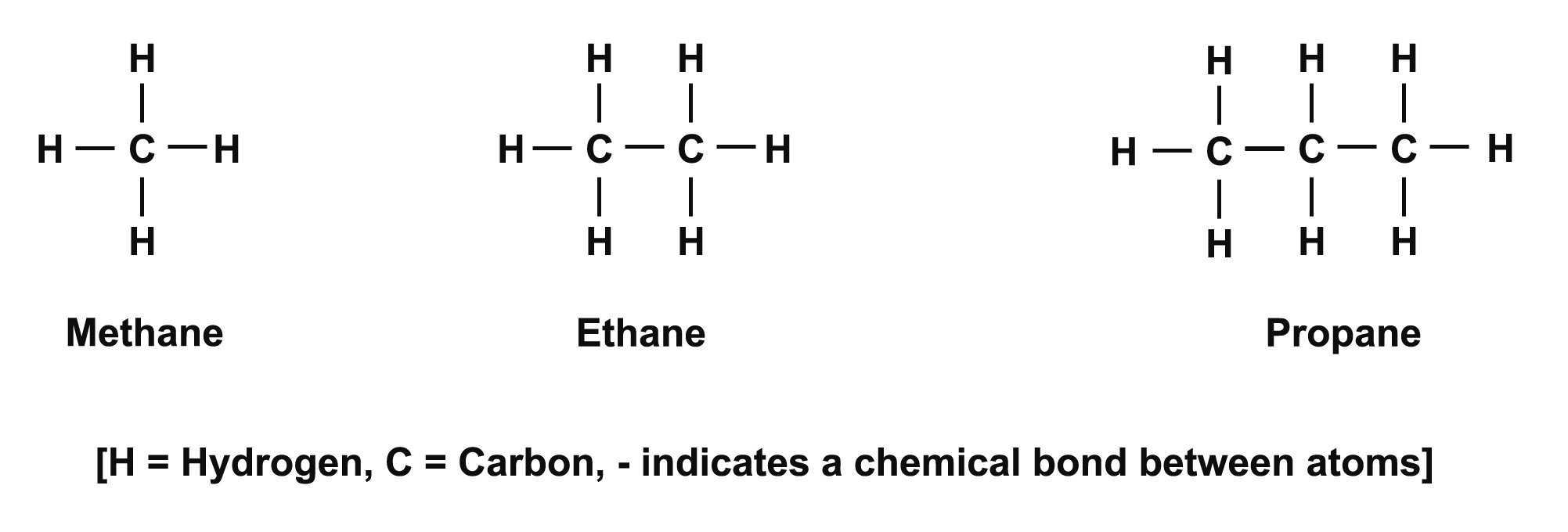
These include a group of organic1 molecules called hydrocarbons that contain only hydrogen and carbon atoms. Examples of the simplest hydrocarbons are shown in figure 1.1.

Figure 1.1. Simple hydrocarbon molecules.
More and more carbon atoms can be added to create large molecules. Such molecules can be found in petrol, kerosene, wax and other oils and plastics.
Non-polar molecules have no electrical charges on them.
These molecules have no overall electrical charge on them but do have small electrical charges at different points. For example water (H20) consists of two hydrogen atoms and an oxygen atom; the hydrogen atoms have a small positive charge and the oxygen has a small negative charge (figure 1.2). The small positive and negative charges attract each other in a similar way to which the north and south poles on a magnet will attract each other. This attraction causes weak bonds to be set up between molecules.
Ethanol (the alcohol that gets you drunk) is another example of a polar molecule. It contains 2 carbon atoms 6 hydrogen atoms and 1 oxygen atom (figure 1.3).
Note that the ethanol molecule has a polar part (the alcohol group) and a non-polar part (the rest of the molecule). In fact whenever you see oxygen in an organic molecule you can assume it to be polar (or at least the part of it that contains the oxygen atoms).

Figure 1.2. The water molecule.
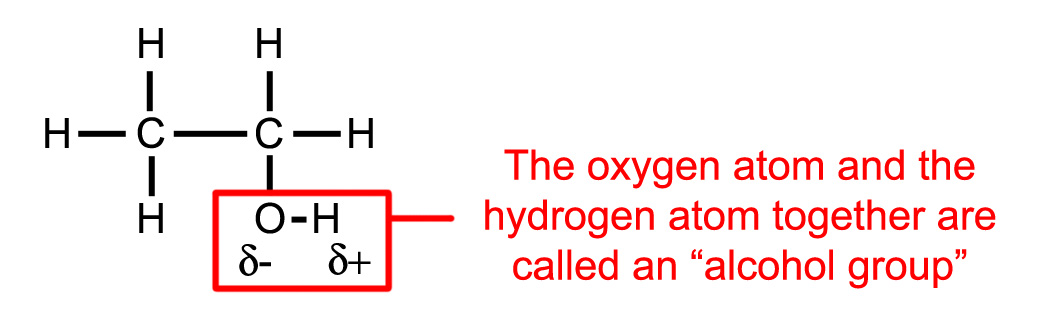
Figure 1.3. The ethanol molecule.
When two liquids will not mix (i.e. their molecules will not interact together freely) they are said to be immiscible; therefore engine oil and water are immiscible. When two immiscible liquids separate the one with the lowest density will rise to the top.
As a rule of thumb like dissolves like. That is to say that polar liquid will mix with other polar liquids but not non-polar liquids, also non-polar liquids will mix well with other non-polar liquids. Liquids that will not mix with water are said to be hydrophobic.
When two liquids are immiscible but do not separate immediately they are said to form an emulsion. Some emulsions are quite stable and will take a long time to separate. For example milk is an emulsion of water and fat but is fairly stable. Other emulsions may separate quite quickly, for example a simple salad dressing of oil and vinegar will separate almost immediately (note that vinegar is water based).
The emulsion itself consists of small droplets of one liquid within the body of a second liquid. An emulsion containing more water than oil is called an oil-in-water emulsion and will consist of small droplets of oil in water. An emulsion containing more oil than water is called a water-in-oil emulsion and will consist of droplets of water in oil.
Emulsions can be stabilised, so that they take longer to separate, by adding an emulsifier (sometimes called a surfactant). These substances tend to have molecules that are polar at one end and non-polar at the other, such molecules can form a “bridge” between the two liquids (figure 1.4).
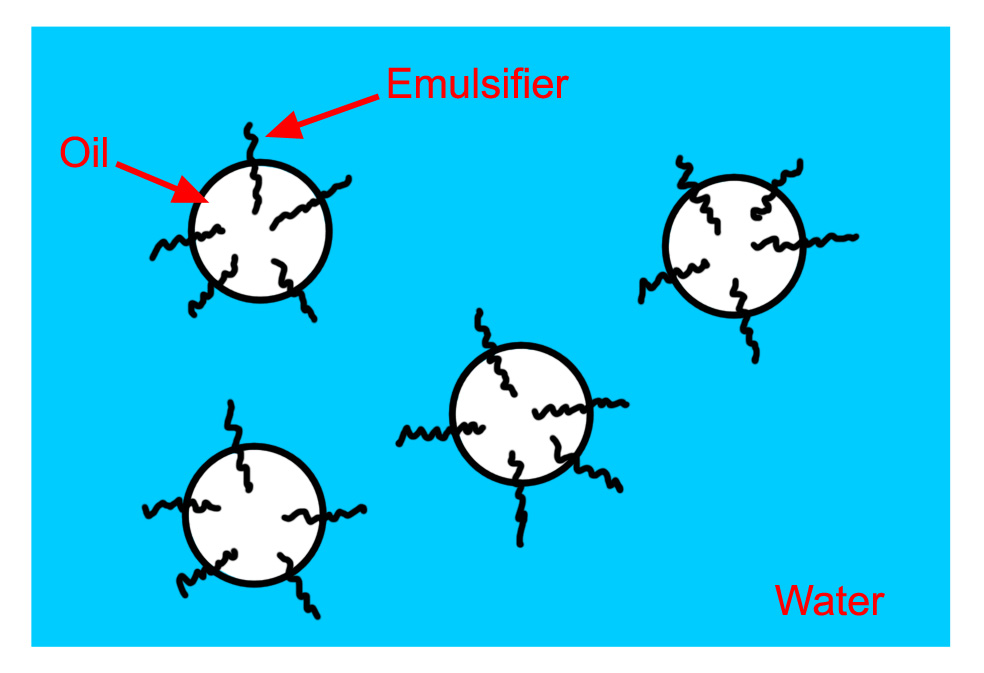
Figure 1.4. A stabilised emulsion.
Oil and water can be made to form a stable emulsion by adding soap. Soap often contains sodium stearate which has a long non-polar “tail” which dissolves in oil and a polar “head” which dissolves in the water (figure 1.5).
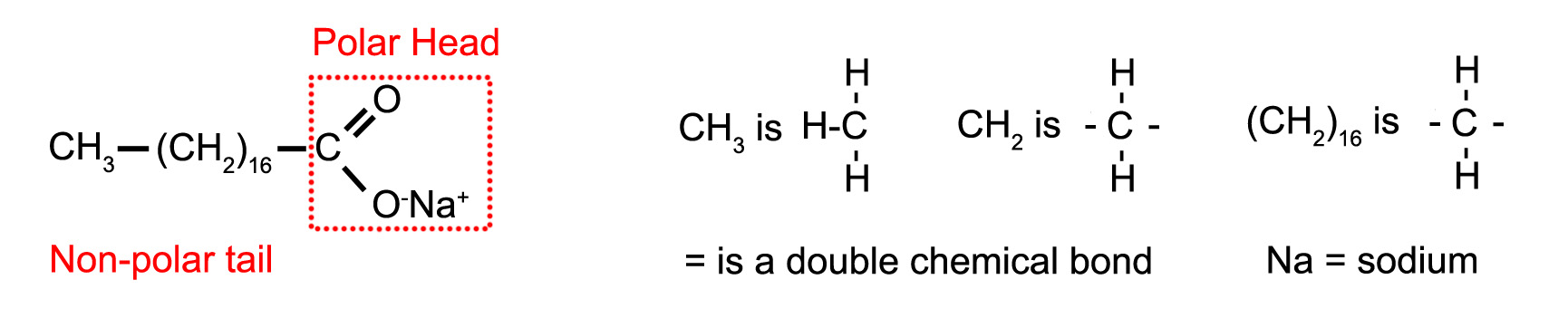
Figure 1.5. Sodium Stearate.
Any molecules with polar and non-polar parts will have some ability to act as an emulsifier. For example alcohol will stabilize an emulsion to some extent, although emulsifiers with longer “tails” tend to work best.
All emulsions will eventually separate. Stable emulsions may stay intact for several years although they will eventually succumb to the forces of gravity. In an emulsion consisting of water and a less dense oil, the droplets of oil will eventually rise to the top and coalesce.
Note: all of the imperial measurements are US values and not UK values (see section A1). The syrup is diluted by about 1:5 with carbonated water when bottled. More information about the ingredients can be found in section 4.
The following recipe is supposedly the original coca-cola recipe that was unearthed in an old notebook that belonged to coca-cola inventor J. S. Pemberton. This recipe was largely substantiated by a second source who had been employed by coca-cola in the USSR during the 1940’s. The recipe and account of how it was found is detailed in the book “For God, Country and Coca-Cola” by Mark Pendergrast (TEXERE, 2000).
|
1 oz Citrate Caffeine |
3 oz Citric Acid |
|
1 oz Ext. Vanilla |
1 Qt. Lime Juice |
|
2.5 oz Flavourings (see below) |
30 lbs. Sugar |
|
4 oz F.E. Coco |
2.5 gal. Water |
Caramel – sufficient
It seems that “F.E. Coco” refers to a fluid extract from coca leaves (the original recipe has the spelling mistake).
|
80 Oil Orange |
40 Oil Cinnamon |
|
120 Oil Lemon |
20 Oil Coriander |
|
40 Oil Nutmeg |
40 Oil Neroli |
1 Qt. Alcohol
There is no indication as to what the numbers preceding the flavour ingredients refer to. However, they may refer to the number of drops. Neroli Oil is an essential oil extracted from the Seville orange tree flower.
Mix Caffeine Acid and Lime Juice 1Qt.
Boiling water add vanilla and flavourings when cool.
Let stand for 24 hours.
Source: www.sodamuseum.com, www.therisenrealm.com
It is suspected that most, if not all of the lime juice has been replaced by lime oil and that glycerine is now added as a preservative (emulsifier). Also, phosphoric acid is now used instead of citric acid. In the USA at least, some of the sugar has probably been replaced with high fructose corn syrup and artificial sweeteners.
The coca extra has also probably been removed (see section 2.2.) and there is no mention of kola nuts from which the drink derives its name. The kola nuts were used for their caffeine content and it is supposed that artificially produced caffeine citrate was used from an early date.
The original recipe was for a drink that was not bottled but sold out of “soda fountains” that where situated inside chemist’s shops. Therefore, the emulsion stability was not as critical as it is for drinks that are intended to be bottle and have a shelf life of several months.
This version is from “Big Secrets” by William Poundstone NY:Quill (1983). This recipe is described by Mark Pendergrast as “a fairly accurate guesstimate” of the modern recipe. The recipe makes 1 gallon of syrup.
Ingredients
|
2.4 kg Sugar, dissolved in the minimum of water |
3.1 g Caffeine |
|
37 g Caramel |
11 g Phosphoric Acid |
|
1.1 g Decocainized Coca Leaf |
0.37 g Kola Nuts |
|
30 g Lime Juice |
19 g Glycerine (vegetarian) |
|
1.5 g Vanilla Extract |
|
Flavourings
|
0.88 g (1.032 ml) Lemon Oil |
Trace Lavender Oil |
|
0.47 g (0.557 ml) Orange Oil |
Trace Neroli Oil |
|
0.20 g (0.190 ml) Cassia Oil |
4.9 g 95% Alcohol |
|
0.07 g (0.077 ml) Nutmeg Oil |
2.7 g (2.7 ml) Water |
|
Trace Coriander Oil |
|
Note: the millilitre (ml) values have been calculated from the weights in grams using the densities in table 4.1. Cassia oil is also known as Chinese cinnamon oil.
Directions
Mix the sugar with just enough water to dissolve it (high fructose corn syrup can be substituted for half the sugar). Add the caramel, caffeine and phosphoric acid. Then add the lime juice (or a solution of water with citric acid and sodium citrate at lime juice strength).
Soak the coca leaf and kola nuts in 22 g of 20% alcohol, strain and add liquid to the mix.
Mix together the essential oils and add the 95% alcohol. Shake. Add the water and let the mixture stand for 24 hours at about 15 deg. C. A cloudy layer will separate. Take off the clear part of the liquid only and add it to the sugar syrup.
Add the glycerine and vanilla extract. Add water (treated with chlorine) to make up to 1 gallon of syrup.
Source: A copy can be found at en.wikipedia.org/wiki/Coca-Cola_formula
Mark Pendergrast adds that this recipe tallies closely with a testimony by a coca-cola chemist at a Delaware court case in 1991: the differences are in the amounts – 13.2 g phosphoric acid (not 11 g), 1.86 g vanilla extract (not 1.5 g) and 91.99 g of single strength commercial caramel (not 37 g). This final difference may be due to a concentrated caramel being used, such products are widely marketed to the drinks industry.
This is supposedly the original Pepsi-Cola recipe. It was submitted to a court in the USA when Pepsi-Cola filed for bankruptcy in 1923.
Ingredients
|
7500 lbs. Sugar – standard confectioners |
12 gal. Caramel – burnt sugar colour |
|
Up to 1200 gallons Water |
12 gal. Lime Juice |
|
58 lbs. Phosphoric Acid – S.G. 1.750 |
|
Flavourings
|
½ gal. Alcohol |
4 fl oz Cinnamon Oil |
|
6 fl oz. Lemon Oil |
2 fl oz Nutmeg Oil |
|
5 fl oz Orange Oil |
2 fl oz Coriander |
|
1 fl oz Petit Grain |
|
Petit Grain is an essential oil extracted from the leaves and twigs from a lemon tree.
Source: www.sodamuseum.com
The people who thought of open source software in the 1970’s released this recipe.
Ingredients
|
2 tsp. 7X formula (see below) |
2.36 kg Sugar – plain granulated |
|
3.50 tsp. 75% Phosphoric Acid or Citric Acid |
0.50 tsp. Caffeine (optional) |
|
2.28 l Water |
30 ml Caramel |
Flavourings
|
3.50 ml Orange Oil |
0.25 ml Neroli Oil |
|
1.00 ml Lemon Oil |
2.75 ml Lime Oil |
|
1.00 ml Nutmeg Oil |
0.25 ml Lavender Oil |
|
1.25 ml Cassia Oil |
10 g Gum Arabic |
|
0.25 ml Coriander Oil |
3 ml Water |
Directions (quoted directly from the original source)
“Mix oils together in a cup. Add gum arabic, mix with a spoon. Add water and mix well. I used my trusty Braun mixer for this step, mixing for 4-5 minutes. You can also transfer to a blender for this step. Can be kept in a sealed glass jar in the fridge or at room temperature. Please note that this mixture will separate. The Gum Arabic is essential to this part of the recipe, as you are mixing oil and water.”
Note: no mention is made as to what you do once the mixture separates.
“In a one gallon container (I used the Rubbermaid Servin' Saver Dry Food Keeper, 1.3 US Gal/4.92 l), take 5 mls of the 7X formula, add the 75% phosphoric or citric acid. Add the water, then the sugar. While mixing, add the caffeine, if desired. Make sure the caffeine is completely dissolved. Then add the caramel color. Mix thoroughly.”
Source: www.colawp.com/colas/400/cola467_recipe.html
This recipe is credited to Dr. Udo Kienle. It uses a sugar substitute but gives more details about the mixing process.
Ingredients (makes 1 litre of syrup)
|
0.07 kg/ l Stevia Natural Sweetener |
6.0 ml/l Salt solution |
|
108.5 ml/l Cola flavor base |
5.0 ml/l Citric acid solution, 50% |
|
8.5 ml/l Phosphoric acid, 85% |
872.0 ml/l Processed water |
|
0.007 kg/l Caramel, acid proof |
|
Flavour Base (makes 1 litre of flavour base)
|
1.32 g/l Vanilla extract |
21.94 g/l Caffeine |
|
4.29 g/l Solid extract Kola nuts |
25.00 g/l Lime oil extract |
|
41.74 g/l Cola flavour emulsion |
75.56 g/l Phosphoric acid, 85% |
|
158.52 g/l Caramel, acid proof |
671.63 g/l Processed water |
Cola Flavour Emulsion (makes 1 litre of flavour emulsion)
|
119.94 g/l Orange oil emulsion |
2.64 g/l Oil of lime, distilled |
|
89.82 g/l Arabic gum |
2.64 g/l Oil of orange, cold pressed |
|
13.21 g/l Oil of cinnamon |
1.32 g/l Oil of coriander |
|
6.60 g/l Oil of lemon, cold pressed |
763.83 g/l Processed water |
Directions
(a). Combine the essential oils (except the orange oil), add magnesium carbonate and filter.
(b). Prepare orange oil emulsion and Arabic gum by heating the orange oil up to 60 deg.C for ½ hour. Remove the oil from the heat and slowly add the Arabic gum. Stir for 15 minutes.
(c). Add the essential oils to the orange oil mixture, then add the water and keep agitating for another 15 minutes.
(d). Homogenize the mixture at 211 kg/cm2.
Notes: there is no mention in the ingredients of magnesium carbonate. Adding magnesium carbonate to water will increases its hardness and will increase the concentration of Mg2+ ions. It is not clear as to the benefit of doing this, although it may refer to using magnesium carbonate as a filtering aid; so that the mixture is filtered through magnesium carbonate.
211 kg/cm2 is the pressure to which a “homogenizer” is set.
Source: www.uni-hohenheim.de/~www440/VTP/stevia/B0/B2
Some people who have performed chemical analysis on Coca-Cola claim that it contains the following ingredients: cinnamon, nutmeg, vanilla, glycerine, lavender, fluid extract of guarana, lime juice and other citrus oils. Coca-Cola themselves admit that their drink contains at least 14 ingredients.
Cola is a water-based drink although it is flavoured with oil. This presents a problem since oil and water do not mix and an emulsifier is needed to create a stable beverage. If the cola has not been sufficiently stabilised the oil and water may separate during storage. Originally coca-cola was not intended to be bottled and stability was less of an issue. The original recipe (section 2.1.) does not contain an effective emulsifier, although alcohol and caramel will have some positive effect.
Caramel, added to cola primarily for its flavour and colour, can also act as an emulsifier. However, it can only make an emulsion of very fine oil droplets stable.
The method from the original recipe (section 2.1.) instructs that the oils should be mixed with ethanol (alcohol) before a little water is added. Essential oils are a complex blend of hydrocarbons and oxygenated organic compounds (see sections 1 and 4.3.). The hydrocarbons have no polarity, will not interact with the alcohol and will separate once the water is added. The compounds that contain oxygen will interact with the alcohol and form a stable mixture with the water. Thus this process creates two phases, a flavour extract (clear part) and a washed oil (cloudy part); the flavour extract is used in the cola.
Treating the oils with alcohol will affect the flavour slightly since some of the molecules in the oils are removed. It also has a stabilising effect because the molecules that are removed are the most hydrophobic and therefore the ones that are most likely to separate from the water during storage.
Several ingredients can be added to give the cola a longer term stability. Coca-cola seems certain to contain glycerine however, Pepsi-Cola’s website does not list glycerine as an ingredient in any of their beverages. There are some rumours that coca-cola had to announce to some Muslim countries (including Morocco) that the glycerine they used was produced from vegetable matter and not from pig fat. Gum Arabic is commonly used as an emulsifier in colas and is included on Pepsi’s ingredient list. New gum Arabic substitutes made from starches are available for use in soft drinks and may be easier to mix.
In addition to emulsifiers, weighting agents can be added to help prevent the oil and water phases separating. These substances are oils with unusually high densities (greater than the density of water). They are mixed with the flavouring oils so that the overall density of the oil phase is the same as the density of water (1 g/ml). Weighting agents therefore stop the oil droplets contained within the emulsion migrating to the surface under the influence of gravity. A common weighting agent is brominated vegetable oil (BVO), however this appears to be banned in Europe and only contained within beverages manufactured in the USA. Even in the USA the amount of BVO that can be added to a beverage is strictly limited.
Glycerol ester of wood rosin (E445,extracted from the stumps of pine trees) is another weighting agent that is sometimes used in citrus flavoured drinks. There are some concerns about the affects that it has on several organs within the human body, it is therefore limited to less than 100 ppm within soft drinks. Other weighting agents suitable for citrus based drinks, such as sucrose acetate isobutyrate, are available from chemical companies.
The densities of various flavouring oils are shown in table 4.1, most are in the range 0.85 g/ml to 0.90 g/ml. An exception is cassia oil, which has a density slightly greater than the density of water; therefore adding more cassia oil will increase the density of the oil phase and increase the stability of the emulsion.
The mixing of the emulsifier with the other ingredients is critical. Often the gum Arabic is first mixed with a small amount of water before it is added to the flavouring oils. Other ingredients, such as caramel and citric acid are usually added as solutions to insure that they mix in easily. For stable emulsions it is usually estimated that the oil droplets should be no bigger that 3 microns2, however if a good emulsifier is not used the droplets should not exceed 1 micron.
Sources:
www.trivia-library.com/c/history-of-the-search-for-the-coca-cola-formula-part-2.htm
www.pepsi.com/help/faqs/faq.php?viewall=yes&category=product_info.
www.caramel.com/solution_center/downloads/caramel.doc
The mineral content of water is important. High levels of minerals such as iron and copper can have an adverse effect on flavour. The stability of emulsions can be reduced if alkaline water (i.e. hard water with a pH greater than 7) is used, therefore calcium and magnesium carbonates should be kept to a minimum. Bottled water is sold with a chemical analysis printed on the label; you should choose the one with the lowest concentrations of Ca+ and Mg2+ ions.
Source: http://www.foodproductdesign.com/ (articles entitled “Pop Art” and “Beverage Stabilizers”)
Sugars are carbohydrates, therefore they contain carbon, hydrogen and oxygen atoms. Normal granulated sugar is sucrose; a sucrose molecule is made from a glucose molecule and a fructose molecule joined together.
Often some of the sugar is replaced with high-fructose corn syrup (HFCS) and artificial sweeteners. There is a small risk that using HFCS will cause the cola to be contaminated with yeast.
These oils are a complex mixture of molecules, some of which dissolve more readily in water and alcohol than others. The constituent molecules can be broken down into three categories:
Hydrocarbons compounds – These are made up from atoms of carbon and hydrogen only. They do not readily dissolve in water but can form an emulsion if an emulsifier is used. Common molecule types include terpenes and monoterpenes.
Oxygenated Compounds – These are organic molecules that contain oxygen. Examples are esters, aldehydes, ketones, alcohols, phenols and oxides. Because they contain polarising oxygen atoms they will be at least partially soluble in water.
Miscellaneous Compounds – Including acids, lactones, sulphur compounds and nitrogen compounds.
It is the hydrocarbons and the oxygenated compounds that contribute most to the oil’s flavour. Different oils will have different amounts of hydrocarbons and oxygenated molecules, therefore different oils will have different solubilities in water and alcohol. Citrus oils have large amounts of terpenes and are not very soluble in alcohol. However the other oils have much larger quantities of alcohols and esters, and are soluble in much smaller volumes of alcohol. The table 4.1 summarises the compositions and solubility of the essential oils used in colas.
As mentioned above some cola recipes suggest that the essential oils are mixed in alcohol. This process will result in the removal of the most insoluble hydrocarbons (probably a large proportion of the terpenes), thus the resulting mixture will form a more stable emulsion with water.
|
Essential Oil |
Composition |
Solubility |
Densitye (g/ml) |
|
Cassia Oila
|
Very similar to cinnamon oil. Contains up to 90% cinnamic aldehyde |
Soluble I part in 2, in 80% alcohol. Will form a clear solution in 3 parts 70% alcohol. |
1.045-1.065 |
|
Coriander Oil |
Largely alcohols with small amounts of terpenes. |
Soluble 2 parts in 1 in alcohol and 1 part in 3 70% alcohol forming a clear solution in the absence of terpenes. |
0.863-0.878 |
|
Lavender Oil |
Chief components are alcohols and esters. The composition varies considerably depending on the source. |
Forms a clear solution in alcohol and in 3 parts of 70% alcohol. |
0.880-0.895 |
|
Lemon Oil |
90% terpenes chiefly limonenec. The lemon flavour is mainly from the oxygen containing compounds such as the aldehyde citral (about 3.5 to 5%). |
Soluble 1 part in 12 alcohol. Probably will not form a clear solution due to wax-like constituents. |
0.851-0.855 |
|
Lime Oil |
Like other citrus oils it is mainly terpenes with smaller amounts of oxygen compounds. |
|
0.910-0.915 |
|
Neroli Oilb |
Odour due to oxygen containing compounds that are present in small quantities. An odourless and tasteless paraffin is also present. |
Soluble 1 part in 11/2 - 2 parts 80% alcohol. Becomes cloudy as the concentration increases. |
0.868-0.880 |
|
Nutmeg Oil |
Contains both hydrocarbons and oxygen compounds but amounts vary depending on source. |
Soluble 2 parts in 1 part alcohol and 1 part in 3 parts 70% alcohol forming a clear solution in the absence of terpenes. |
0.895-0.924 |
|
Orange Oild |
90% limonenec with smaller amounts of aldehydes and esters. |
Soluble 1 part in 7 parts alcohol. A clear solution will not always form because of the presence of waxy non-volatile substances. |
0.842-0.846 |
Table 4.1: The composition and solubility of essential oils. Notes: (a) also known as Chinese Cinnamon; (b) the oil of the orange tree flower; (c) a terpene that occurs chiefly in orange and lemon oils, it is a hydrocarbon with the formula C10H16; (d) orange oil can be from sweet or bitter orange peel, or a mixture of both, while similar, the composition of the two oils vary slightly. (e) Essential Oil densities at 25 deg. C (Source: www.ibiblio.org).
Gum arabic is prepared from the stems and branches of sub-Saharan Acacia senegal and Acacia seyal trees. In colas it is used as an emulsifier. Care must be taken to use a gum appropriate for foodstuffs, also there are “emulsion grade” gums that are specially prepared to be used as emulsifiers in beverages. Gum Arabic is a complex mixture of oxygen containing molecules. Significant amounts of gum are needed to stabilise cola emulsions; somewhere between 18 and 22% weight/volume. Gum substitutes made from starch are now available.
Source: www.lsbu.ac.uk/water/, http://www.foodproductdesign.com/ (articles entitled “Pop Art” and “Beverage Stabilizers”)
Caramel colours are amorphous, brown to brownish materials resulting from the carefully controlled heat treatment of food grade carbohydrates in the presence of small amounts of food grade acids, alkalis or salts. They are often liquids with very fine particles suspended in them (colloid suspensions) and have a density of between 1.25 and 1.36 g/ml.
Caramels have a range of pH3 values and the one that you use should have a low pH (2.5-3.5) that is matched to the acidic environment of the cola itself. You should also check that the isoelectric point is lower than pH 1.5. Caramels that are appropriate to colas are often called “negative caramels”. Failure to choose an appropriate caramel may result in a sediment forming at the bottom of the cola. Since the caramels are so acidic care should be taken when handling them.
Caramel is added primarily for its colouring properties however it will also act as an emulsifier. If caramel is to be the only emulsifier the drops of oil in the emulsion need to be exceptionally small (about 1 micron).
www.caramelworld.com/solution_center/basics_of_caramel_colors.asp
Has the chemical formula HOCH2CH(OH)CH2OH and has a density of 1.26 g/ml. It can be obtained from either animal fat or vegetable matter, therefore you should check if you intent to make a vegetarian cola.
Glycerine is added to the cola as an emulsifier.
Alcohol refers to ethanol that has the formula CH3CH2OH (C2H6O) and has a density of 0.79 g/ml. It is used in some of the recipes to remove the more hydrophobic molecules (terpenes) from the essential oils thus creating a more stable oil/water flavouring mixture.
The alcohol is not later removed from the mixture but once the syrup is completed and subsequently diluted it is present in very small quantities.
A carboxylic acid that is abundant in lemons and limes, making up about 8% of their dried weight. It is used extensively in foods and drinks as a preservative and as flavouring. It has a chemical formula of COOHCH2C(COOH)(OH)CH2COOH (C6H8O7) and has a density of 1.665 g/cm3.
A mineral acid with the formula H3PO4. It is used as an alternative to citric acid and is substantially cheaper to produce in bulk. It has been linked with osteoporosis. It is a liquid with a density of 1.834 g/ml (at 18 deg. C).
Note: The name ‘imperial’ normally refers to the UK values and the US values are usually referred to as ‘US customary units’.
Volume:
1 UK gallon = 4.546 litres
1 US gallon = 3.785 litres
1 UK fluid oz = 28.41 millilitres
1 US fluid oz = 29.57 millilitres
1 UK tablespoon = 15 millilitres (Note: in Australia 1 tablespoon= 20 ml)
1 US tablespoon = 14.8 millilitres
1 UK teaspoon = 5 millilitres
1 US teaspoon = 4.93 millilitres
1 UK quart = 1.101 litres (1/4 US gallon = 2 pints)
1 US quart = 0.946 litres (1/4 US gallon)
Note:
1 litre = 1000 millilitre = 1000 cubic centimetres (cc or cm3) = 100 centilitres (cl)
Mass:
1 ounce = 28.349 grams
1 pound = 0.454 kilograms
Note:
1 kg = 1000 grams
Volume and mass are linked to density by the following simple equation:
density = mass/volume
Therefore to convert from a mass (in grams) to a volume (in millilitres) you must divide the mass by the density. To convert from a volume to a mass you must multiply the volume by the density.
Note: It is essential that you keep the mass, volume and density in the correct units. For example mass in grams, volume in millilitres and density in grams per millilitre.
The density of water is 1 g/ml or 1 kg/l, therefore 1 ml of water has a mass of 1g and 1 l of water has a mass of 1 kg.
|
BVO |
Brominated Vegetable Oil. |
|
deg. C |
Degrees Centigrade. |
|
Ext. |
Extract |
|
F.E. |
Fluid Extract. |
|
fl. oz. |
Fluid Ounce. |
|
g |
Gram. |
|
g/cm3 |
Grams per centimetre squared, a measure of density (1g/ g/cm3=1g/ml). |
|
g/l |
Grams per litre. |
|
g/ml |
Grams per millilitre, a measure of liquid density (1g/ g/cm3=1g/ml). |
|
g/ml |
Grams per litre. |
|
gal. |
Gallon. |
|
HFCS |
High-Fructose Corn Syrup. |
|
kg |
Kilogram. |
|
Kg/cm2 |
Kilograms per centimetre squared, a measure of pressure. |
|
Kg/l |
Kilograms per litre. |
|
l |
Litre. |
|
lbs. |
Pound. |
|
ml |
Millilitre. |
|
oz.. |
Ounce. |
|
pH |
The measurement of acid/base strength . |
|
ppm |
Parts per million |
|
Qt. |
Quart. |
|
s.g. & S.G. |
Specific Gravity. |
|
tsp. |
Teaspoon. |
1 Organic molecules are based around carbon and hydrogen atoms but may contain other atoms such as nitrogen, oxygen and chlorine.
2 1 micron = one 1000th of a millimetre.
3 Acid strength is measured using the pH scale. 1=very acidic, 7=Neutral, 14=very basic (alkaline).