Notes On Making Cola
Section 1
Up to: Cube-Cola
| Contents |
1. Some Basic Chemistry
1.1. Molecules
Molecules are one of the fundamental building blocks of chemical compounds and normally consist of groups of atoms held firmly together by covalent bonds. Molecules generally stay intact during physical processes, such as melting and boiling, but are broken apart and altered during chemical processes or reactions.
Roughly speaking covalently bonded molecules can be split into two basic types:
- Non-polar
- Polar
1.1.1. Non-polar Molecules
These include a group of organic [1] molecules called hydrocarbons that contain only hydrogen and carbon atoms. Examples of the simplest hydrocarbons are shown in Figure 1.1.
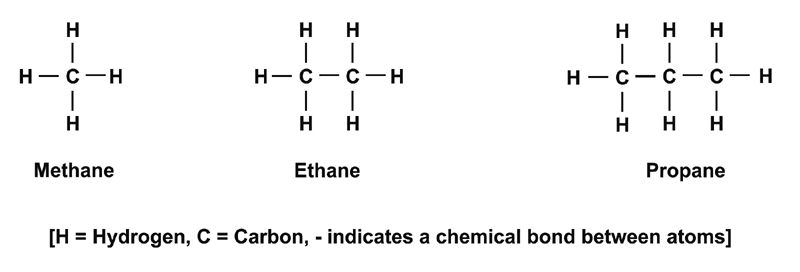
Figure 1.1: Simple hydrocarbon molecules.
More and more carbon atoms can be added to create large molecules. Such molecules can be found in petrol, kerosene, wax and other oils and plastics. Hydrocarbons need not be straight chain molecules (as those depicted in Figure 1.1) they can also be based on hexagon shapes (see Appendix A4 and Appendix A5).
Non-polar molecules have no permanent overall or localised electrical charges on them.
1.1.2. Polar Molecules
These molecules have no permanent overall electrical charge on them but do have small electrical charges at different points. For example water (H20) consists of two hydrogen atoms and an oxygen atom; the hydrogen atoms have a small positive charge and the oxygen atom has a small negative charge (Figure 1.2). The small positive and negative charges attract each other in a similar way to which the north and south poles on a magnet will attract each other. This attraction causes weak bonds to be set up between molecules.

Figure 1.2: The water molecule.
Ethanol (CH3CH2OH, the alcohol that gets you drunk) is another example of a polar molecule. It contains 2 carbon atoms 6 hydrogen atoms and 1 oxygen atom (Figure 1.3). Note that the ethanol molecule has a polar part (the alcohol group) and a non-polar part (the rest of the molecule). Whenever you see oxygen in an organic molecule you can assume it to be polar (or at least the part of it that contains the oxygen atoms). See Appendix A5 for other important oxygen containing groups.
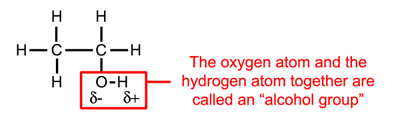
Figure 1.3: The ethanol (CH3CH2OH) molecules.
Ethanol is not the only alcohol. Any molecule that contains C and H atoms and one or more -OH groups is an alcohol. For example CH3OH is called methanol and CH3CH2CH2CH2OH is called butanol.
1.2. Solubility, Miscibility And Immiscibility
When one liquid dissolves in a second their molecules mix freely together and a solution is formed. Such liquids are said to be miscible. When two liquids will not mix (i.e. their molecules will not interact together freely) they are said to be immiscible; therefore engine oil and water are immiscible. When two immiscible liquids separate the one with the lowest density will rise to the top.
As a rule of thumb like dissolves like. That is to say that polar liquids will mix with other polar liquids but not non-polar liquids, also non-polar liquids will mix well with other non-polar liquids but not polar ones. Liquids that will not mix with water are said to be hydrophobic and those that will mix with water are said to be hydrophilic.
Substances are not necessarily soluble or insoluble, in many cases substances are slightly soluble. For example the solubility of alcohols in water is affected by the size of the hydrophobic portion of the molecule and the number of -OH groups present. Hence ethanol (CH3CH2-OH) is completely soluble in water and butanol (CH3CH2CH2CH2OH) is only partially soluble (7.9 g of butanol per 100 ml of water). Also Butanediol (HO-CH2CH2CH2CH2-OH) has two -OH groups and is completely soluble in water although it has the same number of carbon atoms as butanol.
1.3. Acidity And Dissociation
Some polar molecules lose their Hδ+ atoms completely when they dissolve in water. The Hδ+ atom then becomes a free H+ ion [2]. Water molecules themselves are constantly dissociating to produces H+ and OH- ions and the ions in turn are re-associating to produce water molecules:
![]()
Obviously when water molecules dissociate they produce equal numbers of H+ and OH- ions. Crudely speaking an acid is a substance that only produces H+ ions when it is dissolved in water, thus forming a solution with more H+ ions than OH- ions. For example hydrochloric acid (HCl) dissolves in water to give:
![]()
The acidity of a solution is measured using the pH scale which reflects the consecration of H+ ions. A strongly acidic solution has a pH of 1 or 2, a neutral solution has a pH of 7 and a basic (or alkali) solution has a pH between 7 and 14. Bases form OH- ions when dissolved in water (or put another way cause a deficit of H+ ions).
HCl is a strong acid because each molecule will produce an H+ ion. Alcohols on the other hand are very slightly acidic and when a volume of ethanol is dissolved in water a very small proportion of the molecules dissociate:
![]()
Organic carboxylic acids, for example ethanoic acid (CH3COOH, found in vinegar), have a COOH group (see Figure 1.4). When ethanoic acid disassociates in water it produces H+ and CH3COO- ions.

Figure 1.4: The ethanoic acid molecule.
Alcohols and carboxylic acids are both found in essential oils, and while carboxylic groups are stronger acids than alcohols they are both relatively weak acids when compared to hydrochloric or sulphuric acids.
1.4. Emulsions
1.4.1. Types Of Emulsion
When two liquids are immiscible but do not separate immediately they are said to form an emulsion. Some emulsions are quite stable and will take a long time to separate. For example milk is an emulsion of water and fat but is fairly stable. Other emulsions may separate quite quickly, for example a simple salad dressing of oil and vinegar will separate almost immediately (note that vinegar is water based).
The emulsion itself consists of small droplets of one liquid within the body of a second liquid. An emulsion containing droplets of oil in water is called an oil-in-water emulsion and the oil is called the dispersed phase while the water is called the continuous phase. An emulsion with droplets of water in oil is called a water-in-oil emulsion. A good oil-in-water emulsion will consist of very fine oil droplets homogeneously dispersed throughout the body of water.
Since colas are oil-in-water emulsions this discussion of emulsions assumes that an oil phase is being dispersed within a water phase. In practice neither the water phase nor the oil phase of an emulsion are likely to be pure substances. For example in colas the continuous water phase is an acidic solution of citric or phosphoric acid together with other ingredients such as caramel and sugar, the oil phase is a complex mixture of organic molecules from the essential oil flavourings. In general the water soluble molecules all stay in the water phase and the oil phase will be a mixture of all the liquid molecules that are not soluble in water (i.e. a mixture of oils).
1.4.2. Surface Energy
All surfaces have a surface energy, this energy is responsible for phenomena such as surface tension. If you place a drop of oil into a glass of water a new surface at the interface between the oil and water is created and this surface will have an energy (Figure 1.5). This energy must be provided from somewhere, thus to create an emulsion from oil and water you must supply energy. For example you must shake salad dressing to create an emulsion from the oil and vinegar, if you do not shake it the two liquids stay in separate layers (Figure 1.6).
 |
 |
| Figure 1.5: A drop of oil in water. | Figure 1.6: Making new surfaces in salad dressing emulsion. |
Crudely speaking all systems try to reduce their energy to a minimum, thus our drop-of-oil-in-water system wants to reduce the area of the oil-water interface; less surface area equal less surface energy. For a given volume of oil the minimum surface area possible is obtained by forming a sphere; therefore oil drops in water and bubbles of gas in a liquid are always spherical.
1.4.3. Emulsion Failure
Emulsions can fail in four basic ways, each of which causes the homogenous dispersion of oil droplets to be lost (Figure 1.7):
- Coalescence - Two small oil spheres have a combined surface area (and therefore surface energy) that is larger than a single big sphere containing the same volume of oil. Thus if salad dressing is left to stand the small bubbles will coalesce to form bigger and bigger spheres until the oils has completely separated from the water.
- Flocculation - The small spheres of oil stick together to form clumps or flocs which act as if they are larger drops. Therefore the oil is no longer evenly distributed through the water.
- Creaming - Most oils are less dense than water and will therefore float to the top. However, the drops will not necessarily coalesce.
- iv. Breaking - Due to Coalescence and creaming combined, the oil separates completely from the water so that it floats at the top in a single, continuous layer.
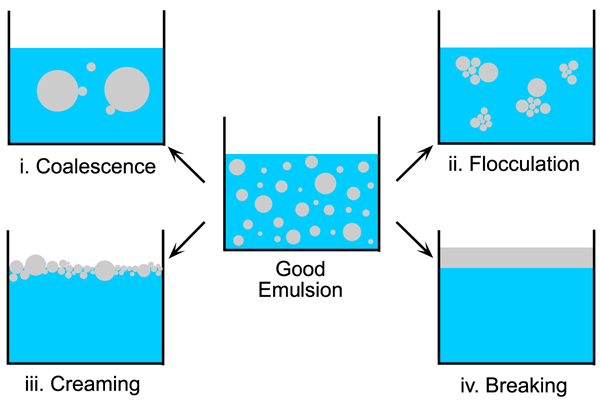
Figure 1.7: The failure of emulsions.
Figure 1.7 clearly shows that flocculation and creaming leave the fine oil droplets intact while making them less well distributed throughout the water. Therefore, these two processes can be reversed by putting in a small amount of energy (i.e. moderate stringing or shaking). Brownian motion, within the water phase, can provide enough energy to keep exceptionally small droplets agitated and hence creaming is less likely to happen with fine emulations. If the emulsion contains larger oil droplets, they will soon rise to the top.
Coalescence and breaking lead to large bodies of oil separating from the water and essentially result in the emulsion separating completely. To reverse this process the emulsion must be remade and this will require a lot of energy. Emulsions are all thermodynamically unstable, meaning that they will eventually separate however, they can be stabilised and in some cases they can remain intact indefinitely. Both coalescence and flocculation are more likely if the surface energy between the two phases is high and if the surface area to volume ratio is high (i.e. the oil droplets are very small). However, emulsions of small droplets are easier to stabilise because creaming is less likely.
1.4.4. The Origins Of Charged Droplets In Emulsions
In many emulsions the droplets of the dispersed phase have an electrostatic charge. In colas the droplets have a negative charge, this arises due to the dissociation of acidic groups at the surface of the droplets (Figure 1.8, also see Section 1.3). These acidic groups form part of some of the oxygen containing essential oil molecules.
The magnitude of the surface charge depends on the strength of the acid groups in the oil phase (for colas the carboxylic groups are quite weak acids and alcohols are very weak acids) and on the pH of the surrounding water phase (for coca-cola this is about 2.5). The more acidic a cola's water phase is, the less surface charge there will be on the droplets and as the pH is lowered a point is reached where the droplets no longer have a surface charge at all, this point is known as the isoelectric point.
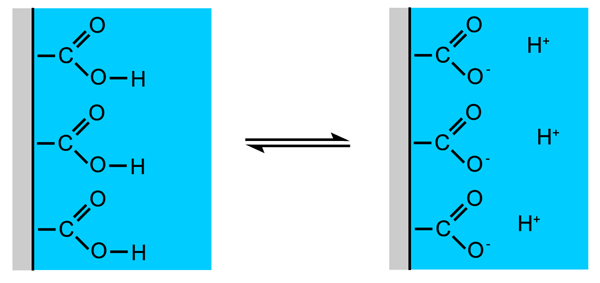
Figure 1.8: Carboxylic acid groups at an oil/water surface producing a surface charge by dissociation.
1.5. Stabilising Emulsions
Coalescence and creaming are constantly working to ruin emulsions however, there are several ways in which emulsions can be stabilised. These include electrostatic repulsion and the addition of additives called emulsifiers and stabilisers [3].
1.5.1. Electrostatic Stabilisation
As mentioned in Section 1.4.4 droplets in emulsions can carry electrostatic charges and these charges are dependent on acidic surface groups and the acidity of the surrounding water phase. Charged oil droplets will repel each other since alike charges repel, this prevents two droplets sticking together or coalescing.
1.5.2. Polymeric Stabilisation
Polymers [4], including natural polymers such as proteins and gums, can be used to stabilise emulsions. Some of these polymers contain certain polar chemical groups, for example alcohols, that can adsorb onto the surface of the oil droplet. Gum Arabic is an example of a polysaccharide gum that can adsorb to oil droplets.
The polymer molecules can either adsorb at a single point or at several points and the adsorbed layer prevents two droplets from coalescing by forming a barrier (Figure 1.9). Molecules that are attached at more than one point form a strong protective barrier around the droplets that can withstand vigorous agitation without becoming detached. These polymer barriers stabilise the emulsion once it has been formed but don't usually make formation easier.
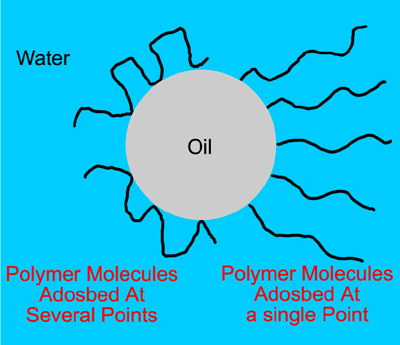
Figure 1.9: Adsorption of polymers at the oil/water interface.
Polymer molecules that can not adsorb onto the oil droplets but are soluble in water can stabilise emulsions by increasing the viscosity [5] of the water phase. Polysaccharide gums such as xanthan gum (but not gum Arabic) can be used to this end and are known as hydrocolloids. For coalescence and creaming to happen the water phase must easily flow around the oil droplets so that intimate contact between the droplets is possible. A more viscous water phase will flow less easily and sometimes cause water to be trapped between droplets that are trying to coalesce (Figure 1.10). Even small quantities of xanthan gum cause a large increase in the viscosity of the water phase.
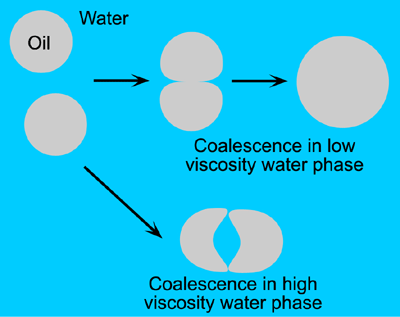
Figure 1.10: Coalescence of oil droplets in water phase.
1.5.3. Small Particles
Very small solid particles can be added to emulsions to act as a stabiliser. The effectiveness of these particles depends on how they interact with both liquids and the interface between them. Like polymeric stabilisation, the particles form a physical barriers around the oil droplets. While small particles can help stabilise an emulsion they probably will not make its initial formation easier.
1.5.4. Surfactants
The name surfactant is short for "surface active agents" and is given to molecules that have an effect on the surface energy of the oil/water interface. These chemicals not only increase the stability of emulsions but also make them substantially easier to form in the first place. Surfactants can achieve this because they act as a 'bridge' between the oil droplets and the water, reducing the surface energy of the interface and thus reducing the amount of energy needed to create new oil/water surfaces (see Section 1.4.2).
Surfactants are molecules that are polar at one end and non-polar at the other, thus one end of the molecule dissolves in the oil droplet and the other end dissolves in the water phase (Figure 1.11).
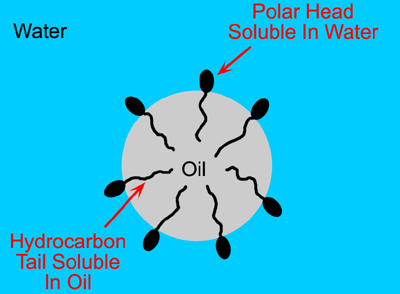
Figure 1.11: The action of surfactants bringing the oil/water interface.
Oil and water can be made to form a stable emulsion by adding soap as a surfactant. Soap often contains sodium stearate which has a long non-polar "tail" which dissolves in oil and a polar "head" which dissolves in the water (Figure 1.12). Any molecules with polar and non-polar parts will have some ability to act as an emulsifier. For example alcohol will stabilize an emulsion to some extent, although emulsifiers with longer "tails" and highly soluble polar "heads" tend to work best.
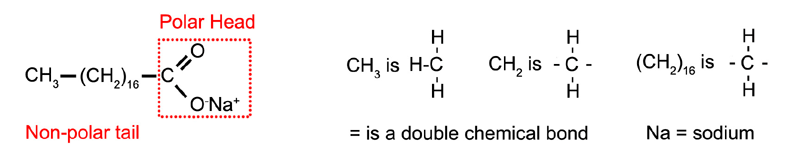
Figure 1.12: Sodium stearate.
1.5.5. Weighting Agents
Oil-in-water emulsions will eventually separate because most oils are less dense than water and will eventually float to the surface. This process is called creaming (see Section 1.4.3) and can be delayed if the oil droplets are very fine. Creaming will not happen if the oil phase has the same density as water.
Weighting agent are oils that, unusually, have a density greater than the density of water. Thus the overall density of the oil phase can be increased by add such a substances. The closer the density of the two phases, the more stable an emulsion will be.
| Contents |
[1] Organic molecules are based around carbon and hydrogen atoms but may contain other atoms such as nitrogen, oxygen and chlorine. [Return To Text]
[2] As mentioned in Section 1.1.2 polar molecules have no overall charge. However, if a water molecule splits up it forms an H+ ion , this is a hydrogen atom with a an electron missing and therefore it has a positive charge. An OH- ion is also formed, which is an oxygen atom and a hydrogen atom joined together with the extra electron, it therefore has a negative charge.[Return To Text]
[3] The terms emulsifier and stabiliser are often interchangeable however, emulsifiers are strictly additives that make the formation of emulsions easier and stabilisers are additives that prevent the failure of the emulsion once it has been mixed.[Return To Text]
[4] Polymers are long molecules made up from a repeated pattern of smaller molecules stuck together.[Return To Text]
[5] Viscosity is a measure of the ability of a liquid to flow, therefore treacle has a higher viscosity than water.[Return To Text]